All plasma collected in Italy comes from voluntary, periodic, responsible, anonymous and unpaid donations. Each Region sends the plasma collected at the Blood Establishments in its territory to the authorised contracted company to process it for the production of PDMPs.
All plasma collected in Italy comes from voluntary, periodic, responsible, anonymous and unpaid donations. Each Region sends the plasma collected at the Blood Establishments in its territory to the authorised contracted company to process it for the production of PDMPs.
The contract with companies acting as service providers, is regarded as “toll fractionation”, which stands as a formal agreement for the production of PDMPs.
Contracts are awarded following a tender procedure in accordance with current regulations. In Italy, four inter-regional agreements have been entered into force for the acquisition of the service:
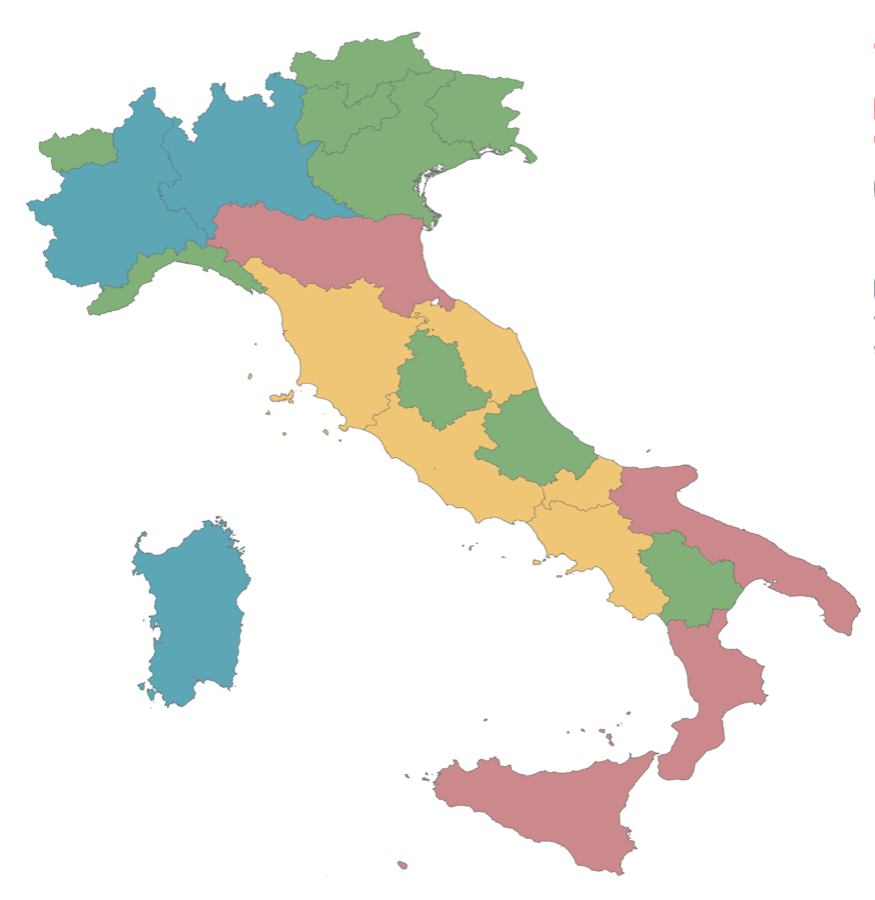
- The Lombardy-Piedmont-Sardinia Agreement (L.P.S.), with Lombardy as lead Region
- The New Inter-Regional Agreement for Plasma/Plasma derivatives (NAIP), with the adhesion of Abruzzo, Basilicata, Friuli V. Giulia, Liguria, the Autonomous Provinces of Bolzano and Trento, Umbria, Veneto (lead Region), and the Aosta Valley
- The Interregional Plasma/Plasma Derived Products Cluster (RIPP), whose members are Calabria, Emilia-Romagna (lead Region), Apulia and Sicily
The Plasma Network (PLA. NET) comprising Campania, Latium (including the Inspectorate General for Military Health), Marche, Molise and Tuscany (lead Region)
Under the terms of this type of agreement, as set out in the Ministerial Decree of 12th April 2012, the production of PDMPs is regulated by a qualitative-quantitative production plan. The company concerned undertakes to produce the set quantity and guarantee the quality of the PDMPs requested by the Regions and ensures compliance with the planned timeframe and the specified procedures. The contracting Regions, on their part, undertake to make the required plasma available according to the agreed quantity and quality specifications.
The Regions have the right to full ownership of the plasma sent for industrial processing, of any pharmaceuticals deriving from it, and of the residual material. Therefore, the supplier of the processing service may not use the plasma, intermediate fractions or finished products, nor residual raw material, for purposes other than those provided for in the agreement, without prior consent from the Regions.
New interregional agreements 2023
11.0 kg – 1000 pop16.1 kg – 1000 pop16.0 kg – 1000 pop17.6 kg – 1000 pop